Ex 5.53 - Variation of the Equilibrium Constant
The relation between standard reaction Gibbs free energy and the equilibrium constant of the reaction is given by
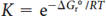
Where ΔGro is the standard reaction Gibbs free energy (kJ·mol-1), T is the temperature (kelvin), and R is the gas constant (J·K-1·mol-1).
To graph the variation of the equilibrium constant click New Plot. Up to 5 plots can be displayed at one time. The Clear button will remove all plots.To see the parameters for each plot click the Legend button. The Redraw button will refresh the graph. This is useful when the function domain (i.e., T ) has been changed. To see the value of each plot at a given point, move your cursor to the desired location then click.