Fig 7D.2 - Variation of Rate Constant with Temperature
The general form of an Arrhenius plot of ln kr against 1/T is given by the equation below. The slope is equal to -Ea/R and the intercept at 1/T = 0 is equal to ln A.
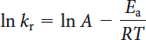
Where A is the pre-exponential factor (same units as kr) and kr has units of s-1 (for a first order reaction), T is the temperature (kelvin), R is the gas constant (kJ·K-1·mol-1), and Ea is the activation energy (kJ·mol-1).
To graph the variation of the rate constant kr enter the parameters (i.e., A, Ea) and click New Plot. Up to 5 plots can be displayed at one time. The Clear button will remove all plots. To see the parameters for each plot click the Legend button. The Redraw button will refresh the graph. This is useful when the function domain (i.e., T ) has been changed. To see the value of each plot at a given point, move your cursor to the desired location then click.