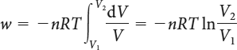
Fig 4B.3 - Work of Reversible, Isothermal Expansion of an Ideal Gas
For an ideal gas, the work of isothermal reversible expansion is equal to the area below the curve of the graph of pressure plotted against volume.
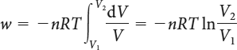
Where V1 and V2 are the initial and final volumes, n is the amount of gas molecules (mol), T is the temperature (kelvin), and R is the gas constant (J·K-1·mol-1).
Note that, for a given change of volume and fixed amount of gas, the work is greater the higher the temperature.
To calculate work (kJ) enter the n, T, and initial and final volumes and click Plot.