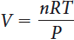
Fig 3B.4 - Charles's Law ( V vs. T )
The ideal gas law is given by
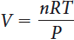
Where P is the pressure (kPa, bar, atm, Torr), n is the amount of gas molecules (mol), T is the temperature (kelvin), and R is the gas constant.
To graph volume (liters) enter n and P and click New Plot. Up to 5 plots can be displayed at one time. The Clear button will remove all plots. To see the parameters for each plot click the Legend button. The Redraw button will refresh the graph. This is useful when the function domain (i.e., T ) has been changed. To see the value of each plot at a given point, move your cursor to the desired location then click.