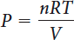
Fig 3B.2 - Boyle's Law
For a fixed amount of gas held at a constant temperature, the volume decreases as the pressure is increased.
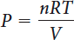
Enter the desired sample amount (n, moles) and temperature (T, kelvins) and choose the range and increments of volume (V, liters) to plot, then click New Plot.
Up to 5 plots can be displayed at one time. The Clear button will remove all plots. To see the parameters for each plot click the Legend on/off button. The Redraw button will refresh the graph. To see the value of each plot at a given volume, move your cursor to the desired location then click. To remove the point click again.