Fig 7B.6 - Test for a Second Order Reaction
(and Time Dependence)
The variation with time of the concentration of a reactant in a second-order reaction is given by
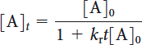
Where [A]0 is the initial concentration of reactant (mol·L-1), t is time (s), and kr is the rate constant (L·mol-1·s-1). Taking the inverse of each side of this equation gives
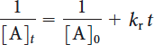
where kr is the slope, and 1/[A]0 is the intercept, of the best fit line.
To calculate the rate constant enter data pairs (t, [A]t) in the appropriate units, and then click the Update button. To see a value on the graph, move your cursor to the desired location then click.
|
1 / ([A]t / units) Show least-squares line Show grid |
|