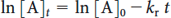
Fig 7B.2 - Determination of Rate Constant
(First-Order Rate Law)
The integrated form of the first-order reaction rate law can be written as
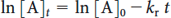
Where t is time, [A]t is the concentration of species A, and kr is the rate constant of the reaction.
The data are expected to give a straight line when ln([A]t) is plotted against t. Note, any experimental parameter proportional to concentration (e.g., partial pressure (Torr), absorbance, fluorescence intensity, etc.) can be substituted for concentration in the analysis of kinetic data. The slope of this line gives the rate constant as -kr.
To graph the best fit line enter the (t,[A]t) data pairs in the appropriate units and click Update. To show a value on the graph, move your cursor to the desired location then click.
|
Show least-squares line Show grid |
|